Pharmaceutical & Biotechnology
Ultrafiltration
Ultrafiltration (UF) is a type of membrane filtration in which pressure and concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate).
The main purpose of tangential flow filtration in bioprocessing is to concentrate a product previously filtered in an earlier step in the production process. The feed solution is circulated in a closed loop, passing through the filter.
This filter is selected to provide retention of the product being concentrated while allowing unwanted/excess buffer and/or background material in the circulating solution to permeate through the filter material to drain away.
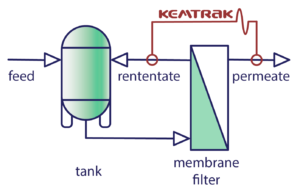
Benefits include:
- Real time continuous measurement
- Non-invasive analytical method
- Processes Optimization
- Quality control
- Concentration monitoring
- Dual-wavelength measurement
- Zero cell hold up volume
Ultrafiltration Application Note
DCP007-UV Product brochure
TC007 Product brochure
The Kemtrak DCP007-UV LED photometer uses a state of the art high performance UV LED light source and robust fiber optics. The sample is subjected to ultra-low power cold light of the exact wavelength required for the analysis, thousands of times less powerful than that used on traditional photometers. The Kemtrak UV LED light source is also modulated and can be configured to measure during very short pulses, further reducing the exposure of the sample to harmful UV radiation.
The Kemtrak TC007 LED Turbidimeter uses high performance long life NIR LED lamps to accurately measure the turbidity of the sample in accordance with the International Standard ISO7027 defining turbidity measurement.
Kemtrak measurement cells are available with FDA and UPS Class VI approved seal materials. The zero dead-volume design assures a fast response and minimize risk of cross contamination. A NIST validation accessory is available to verify analyzer performance without process interruption.
Contact a Kemtrak sales representative for more information.
