Pharmaceutical & Biotechnology
Centrifuge control
Centrifugation is a process which involves the use of centrifugal forces for the sedimentation of heterogeneous mixtures, it uses separation by means of accelerated gravitational force achieved by rapid rotation. This can either replace normal gravity in the sedimentation of suspension or provide the driving force in the filtration through a filter medium of some kind.
Production of bulk drugs
After crystallization the API are separated from the mother liquor by centrifugation.
Production of biological products
Most of the biological products are either proteinaceous or macromolecules. During manufacturing they remain in colloidal dispersion in water. By normal methods of filtration, it is difficult to separate the colloid particles. In those cases, centrifugal methods are used.
Evaluation of suspensions and emulsions
To enhance the rate of sedimentation and creaming the suspension or emulsion is introduced in a centrifuge and rotate at an rpm of 200 to 3000. If still the problems do not appear then the suspension or emulsion can be taken as stable formulation.
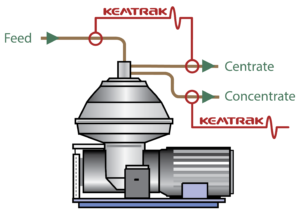
Benefits of a Kemtrak Instrument include:
- Real time continuous measurement
- Optimize centrifuge operation
- Increase product yield / reduce product loss
- Optimize centrifuge operation
- Quality control
Centrifuge control Application Note
The Kemtrak TC007 turbidimeter uses a long life NIR LED light source to precisely measure the optical density of turbid liquids for applications such as monitoring the solids concentration in the concentrate. With the NBP007 Extreme Suspended solids analyser up to very high suspended solids
Optic fibers are utilized to pipe the light from within the analyzer enclosure to the sampling point and back. Kemtrak measurement cells are available in a range of materials and design, including DIN, ASME and sanitary TriClamp flanges.
Kemtrak hygienic measurement cells available with FDA and UPC VI approved sealing materials, convenient zero dead volume design and contain no electronics or moving parts for ease of use.
Contact a Kemtrak representative for further information.
